Viral
Carrier Status is Instilled by Viral Regulatory Particles
Rand
Wheatland
The
Endocrine Research Project,
574 Sims Rd., Santa Cruz, CA 95060, USA
E-mail: rwheatla@query.com
Originally
published in the journal Medical Hypotheses, 2010;74(4):688-91.
But this HTML version has color figures.
Summary
Human viral carriers are important agents in the periodic resurgence
of many pathogens. Instillation of virus in human carriers explains
several of the unusual epidemiological features of viral epidemics,
such as where viruses linger between epidemics and how epidemics can
arise without an apparent source. By inactivating itself, a virus can
easily reside in a host for months or years without being noticed by
the immune system, enabling the virus to be dispersed inconspicuously
in the future and into new regions. When this silent activity of
human carriers is appreciated, it is easier to understand the
dynamics of viral epidemics, such as the explosive appearance of
influenza epidemics.
During viral
illnesses, virus in infected cells is put into a latent state by
regulatory sequences delivered by particles produced by other
virus-infected cells. These regulatory particles are similar to the
virus's virion but contain specific subsets of the viral genome and
cannot replicate in cells that are not infected by the complete viral
genome. Regulatory particles have previously been referred to as
defective interfering particles, noninfective viruses, inactive
viruses, incomplete viruses, satellite viruses, and defective
viruses.
There are still many
unanswered questions regarding viral carrier creation and the role
human carriers play in the pathology and epidemiology of viral
diseases. Some of these questions are presented and discussed in
relation to regulatory particles, possible investigations and how
carrier status may affect the health of the carrier.
Viral
regulatory particles limit the extent of viral infections and shift
the active infection to a latent infection. Just as multicellular
creatures use hormones as chemical messengers to coordinate cellular
functions, viruses utilize regulatory particles to coordinate viral
modes among infected cells within a host. Many viruses depend on
these particles for their continued existence. If we wish to
comprehend and effectively treat viral infections, we must secure a
thorough understanding of viral regulatory particles.
Human viral carriers
are important agents in the periodic resurgence of many pathogens
(1). A viral carrier is a host who is
persistently infected by a virus that exhibits little or no activity,
except when the virus blooms to disseminate viral seeds. This
condition is also described as a latent infection.
Instillation of
virus in human carriers explains several of the unusual
epidemiological features of viral epidemics, such as where viruses
linger between epidemics and how epidemics can arise without an
apparent source. Influenza epidemics have been well studied and are a
good example of these mysteries. Influenza A and B are seasonally
epidemic, generally appearing during the cold season, or the rainy
season in the tropics (2). Few human influenza
infections are observed during the interval between epidemics. When
influenza does appear, it spreads quickly over large regions but may
slowly diffuse over relatively small distances (3).
Widespread latent infection of human carriers allows influenza to be
widely and simultaneously disseminated when awakened by an
environmental trigger. No other mechanism satisfactorily explains the
explosive appearance of an influenza epidemic after a prolonged
absence.
By inactivating
itself, a virus can easily reside in a host for months or years
without being noticed by the immune system, enabling the virus to be
dispersed inconspicuously in the future and into new regions.
Although their activity is not readily apparent, the source of many
viral epidemics can be attributed to asymptomatic human carriers who
are occasionally infectious. For example, for hundreds of years,
ship's surgeons have noted that influenza epidemics have begun on
ships that have been at sea for weeks with the entire crew being
healthy until the epidemic began (4). Influenza
epidemics have also been reported that began in isolated communities
soon after a ship (5) or a plane (6)
arrived even though no one on board appeared to be suffering from the
disease before or during the epidemic. Clearly, human viral carriers
were the source of these epidemics.
Influenza epidemics
tend to be dominated by a single strain. A wave of influenza
infections by a new, nondominant strain of virus is called a herald
wave (7,8). Herald waves
can happen at any time but are most noticeable at the end of the
influenza season, when physicians still have a high suspicion for an
influenza diagnosis and the new strain represents a large proportion
of influenza cases. An excellent example of a herald wave is the
novel strain of H1N1 influenza virus that circulated widely from
March to July, 2009 (9). The influenza
surveillance data in Fig. 1 shows a similar H1N1 herald wave that
occurred in Japan from March to July, 1986. During a herald wave,
carriers are seeded with the new influenza strain, positioning the
virus so it can initiate an epidemic. If the herald wave is able to
infect many hosts, it is a sign that the dominant strain's influence
is waning and that the herald strain will probably supplant the
dominant strain in the next epidemic. The herald wave only makes
sense when the role of human viral carriers in starting influenza
epidemics is appreciated.
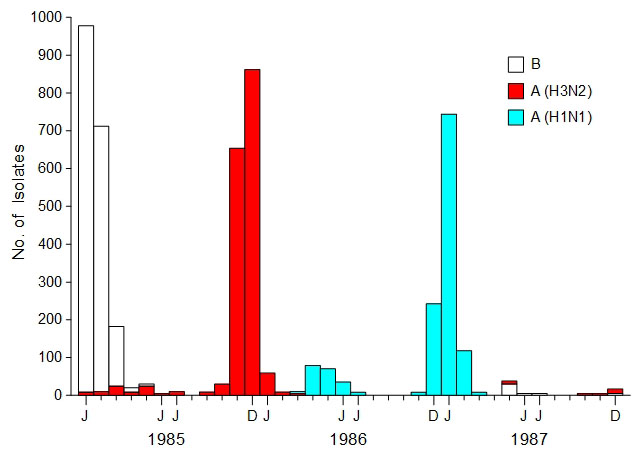
Fig.
1. Influenza surveillance, Japan
(1985-1987). Data represents the total number of isolates per month
that were identified by strain as reported to the National Institute
of Health in Japan. [Adapted from the Annual Report of National
Epidemiological Surveillance of Vaccine-Preventable Diseases, 1992,
Fig. 4 and Nakajima et al. (10), Fig. 1]
Periodic infectious
activity is an efficient alternative to perpetual viral transmission.
This strategy conserves and allows time for renewal of the pool of
susceptible hosts (11). In addition, a virus
gains much more mobility by silently residing in its host. If a virus
could only spread from an ill host within a short period after
infecting the host, the distance it could travel from the point of
infection would be limited. However, if a virus establishes a latent
infection, when it awakens later to spread its virions, it may have
traveled thousands of miles. Since the virus is not confined to a
single community, its long-term survival chances are vastly improved.
Viral Carrier
Creation
How are human viral
carriers created during viral illnesses? Virus in infected cells is
put into a latent state by regulatory sequences delivered by
particles produced by other virus-infected cells. These regulatory
particles are similar to the virus's virion but contain specific
subsets of the viral genome and cannot replicate in cells that are
not infected by the complete viral genome (12,13).
When the complete virus and its regulatory sequence are coresident in
a cell, the virus makes fewer virions and more regulatory particles.
Essentially, the virus goes to sleep while communicating to other
virally infected cells to do the same. The virus uses this method to
coordinate a change in modes after reaching an adequate infection
level.
A virus can be quite
successful if it can latently infect carriers who will spread the
virus to begin new epidemics and possibly introduce the virus to new
areas when their host travels. To accomplish this, the virus must
avoid killing its host and delay becoming latent until enough cells
are infected so that, when awakened, it will be able to produce
abundant viral shedding before being stopped by the immune system.
Viruses do this by disseminating regulatory particles. Regulatory
particles have no effect on virion production unless their regulatory
sequences are in cells that are infected with the complete virus.
Therefore, viral infections do not create many latently infected
cells until virus-infected cells and regulatory sequence-containing
cells have each reached a density threshold. As more infected cells
contain the regulatory sequence, the number of regulatory particles
increases substantially, which should bring the infection to an
abrupt end and also keep the virus from killing its host. Fig. 2
illustrates the transition from an active infection to a latent
infection on a local scale by a series of snapshots of the infectious
state of the same cells over time.
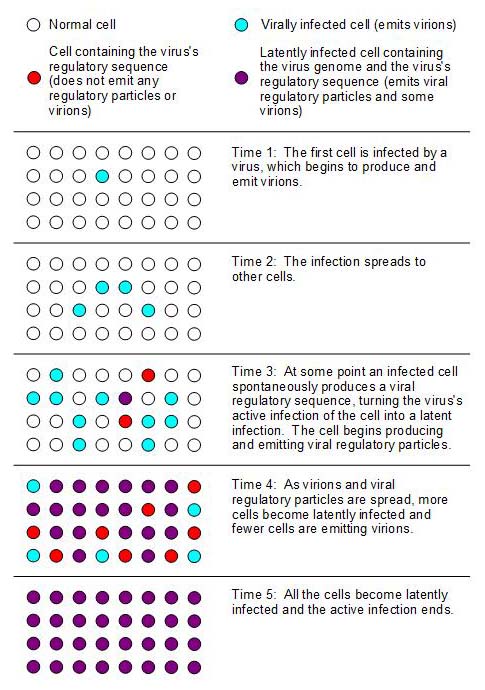
Fig.
2. The dynamics of viral regulatory particles
During the 1940s and
1950s, it was noticed that virus was difficult to grow when the
culture was started with concentrated inocula that had come from
extended incubations (14,15).
Also, after several undiluted passages in cell or tissue cultures,
the harvested fluid lost its infectability in hosts (14,15).
Researchers isolated the particles responsible for the interference
with the culture growth and described their properties: their
proteins and antigenicity were similar to virions and the particles
inhibited the propagation of the virus (13,16).
Later, these particles were inappropriately named defective
interfering particles (12). They should
properly be called regulatory particles because they are not
defective and they perform more than an interfering function. These
particles have also been referred to as noninfective viruses,
inactive viruses, incomplete viruses, satellite viruses, and
defective viruses. Regulatory particles are not viruses or viral
seeds. They are viral tools. They can implant themselves in a cell
but they cannot reproduce without viral assistance. Therefore, they
cannot be said to infect cells.
Although the
regulatory function of regulatory particles was discovered over forty
years ago, the importance of these particles in the pathogenesis and
epidemiology of viral diseases has not been recognized. There are
many fundamental questions regarding viral carriers and viral
regulatory particles that need to be investigated. Several of these
questions vital to understanding viral infections are:
Does every
individual ill from a viral infection become a carrier?
If a virus produces
regulatory particles, which most viruses appear to produce (16-19),
do a few cells always become latently infected during a viral
illness? How many latently infected cells are required to become an
effective viral carrier? If becoming an effective carrier is not a
certainty after surviving a viral illness, naturally infected animals
can be studied to investigate whether there are factors that support
the instillation of carrier status, factors such as season,
nutrition, age, gender, crowding, and illness severity. For instance,
do these factors alter the rate of spontaneous generation of viral
regulatory particles?
Do latently
infected carriers ever clear the virus?
Some viral carriers
may clear their virus upon the first awakening or bloom of the virus.
Other carriers may carry and occasionally spread the virus over their
remaining lifetime. Does the duration of the carrier state depend
more on a person's immune system or on the particular virus? If the
carrier status for a virus is not permanent, can the virus be cleared
while it is latent, as it assumes a very low profile, or must the
host wait for the virus to bloom before the host's immune system can
act? Upon awakening, how well does the immune system detect the virus
and how long does the carrier shed virions?
What fraction of
the population are carriers of each type of virus?
To understand the
epidemiology of viruses it would be helpful to know the prevalence of
carriers for each virus type, by age. Since carrier status may
influence mortality, this bias in estimating prevalence can be
minimized by performing a survey of people that have died after
accidents. PCR analysis of samples should be able to identify viral
carriers and determine which organ, gland or cell type is the viral
reservoir. The study's utility can be increased by searching for many
different viruses in each sample (20).
What triggers a
latent virus to awaken?
Some environmental
factors that have been proposed as triggers for carrier-provoked
epidemics are day length, changes in temperature or humidity and
stress, resulting from situations such as crowding or travel. Records
of ships at sea that have reported epidemics starting on board at the
same time as epidemics arose on the coast that they were passing (4)
is evidence that local meteorological conditions can stimulate latent
infections to awaken.
Most environmental
triggers must awaken latently infected cells via normal
physiochemical processes. A latently infected lung epithelial cell
does not respond directly to the length of the day, the cell only
notices changes in its local chemical milieu. Therefore, to
understand how a latent virus blooms, its environmental trigger and
associated cellular activator should be identified.
Since cells in vitro
can be latently infected using a mixture of virions and regulatory
particles (21-23), it is relatively simple to
conduct experiments into the chemical factors necessary for
interrupting latency. Latently infected cells can be challenged by a
number of different chemical concentrations, combinations and
concentration transitions to determine which stimulus will restore
the latently infected cell's virion production. Obvious environmental
factors to test are: length of day (using vitamins and hormones that
demonstrate seasonal differences, such as vitamin D and melatonin)
and stress (via stress hormones, such as cortisol and ACTH).
Does the
awakening of a latent virus affect the carrier?
When a carrier's
latent viral infection awakens and the virus is being spread, the
carrier does not usually appear to be sick. Although the carriers are
said to be asymptomatic, do they still suffer from some symptoms that
are not notable because the symptoms are mild, common, alterations of
mood or are of a short duration? Symptoms such as being sleepy,
fatigued, depressed or irritable may occur when a carrier's virus is
blooming but are ignored because, normally, these symptoms are not
complained about or clinically acknowledged until the symptom becomes
chronic and sufficiently severe. When a latent infection blooms, to
maximize its transmission potential, it needs its host to associate
with as many contacts as possible. Since a person with severe
symptoms will often isolate themselves and people tend to avoid a
sick person, the infection will encounter many more possible victims
if its host does not appear to be ill. Therefore, the awakening of a
latent infection may have some negative effects on its host's
well-being but if the infection refrains from inducing obvious signs
of illness, it can spread to numerous casual contacts.
The blooming of a
latent viral infection may be responsible for the condition known as
Seasonal Affective Disorder (SAD). SAD is major depression that
recurs seasonally. In the most common type of SAD (winter type)
patients experience symptoms of atypical depression during the fall
and winter, including excessive sleepiness, increased appetite,
carbohydrate craving and weight gain, and attain full remission
during the spring and summer (24,25).
For many winter type SAD patients, daily exposure to bright
artificial light is an effective treatment for avoiding or
significantly decreasing depressive symptoms during the fall and
winter (24,25). Does this
imply that the symptoms of SAD can be avoided if the latent
infection's environmental trigger is nullified?
Which is most
important for the normal termination of a viral infection, viral
regulatory particles or the immune system?
To a sophisticated,
well-adapted virus, the immune system as opposition may just be a
nuisance. In this case, does the virus control the peak level of
infection, becoming latent when it reaches its programmed threshold?
By limiting its spread within its host, a virus protects the host
from superinfection, ensuring the survival of its carrier.
In addition to the
self-regulation mediated by regulatory particles during a viral
illness, is carrier status partially responsible for immunity
following the illness? Upon reinfection with a virus that responds to
the same regulatory particles, the virus can hardly reproduce in such
a host since many of the virus's target cells are already implanted
with regulatory sequences. Similarly, are the regulatory particles in
live vaccines, such as the measles (26), oral
polio (27) and intranasal influenza vaccines,
responsible for the vaccines' effectiveness? If regulatory particles
play a role in resistance, is waning immunity due to decreasing
levels of latently infected cells?
Since regulatory
particles block the spread of virions within an infected host, can
these particles be used therapeutically for terminating severe viral
infections? If regulatory particles can be an effective therapy, this
treatment should be used with caution. Creating an unnatural latent
infection in a patient may cause serious problems later.
Conclusion
Viral regulatory
particles limit the extent of viral infections and shift the active
infection to a latent infection. Just as multicellular creatures use
hormones as chemical messengers to coordinate cellular functions,
viruses utilize regulatory particles to coordinate viral modes among
infected cells within a host. Many viruses depend on these particles
for their continued existence. If we wish to comprehend and
effectively treat viral infections, we must secure a thorough
understanding of viral regulatory particles.
Conflicts of
interest statement
None declared.
References
1.
Novy FG. Disease carriers. Science
1912;36(914):1-10.
2. Shek LP, Lee BW.
Epidemiology and seasonality of respiratory tract virus infections in
the tropics. Paediatr
Respir Rev 2003;4(2):105-11.
[Abstract]
[Free
Full Text]
3. Shope RE.
Influenza: history, epidemiology, and speculation. Public
Health Rep 1958;73(2):165-78.
[Citation]
[Free
Full Text]
4. Hirsch
A. Handbook
of geographical and historical pathology.
(translated by Charles Creighton). London: New Sydenham Society,
1883:18-41. [Free
Full Text]
5. Brown P, Gajdusek
DC, Morris JA. Epidemic A2 influenza in isolated Pacific island
populations without pre-epidemic antibody to influenza virus types A
and B, and the discovery of other still unexposed populations. Am
J Epidemiol 1966;83(1):176-88.
[Citation]
6. Burnet FM. Virus
as organism: Evolutionary and ecological aspects of some human virus
diseases. Cambridge, MA: Harvard University Press, 1945:105.
7. Glezen WP, Couch
RB, Six HR. The influenza herald wave. Am
J Epidemiol 1982;116(4):589-98.
[Abstract]
8. Moriuchi
H, Oshima T, Komatsu S, et al. The herald waves of influenza virus
infections detected in Sendai and Yamagata cities in 1985-1990.
Microbiol
Immunol 1991;35(5):375-88.
[Abstract]
9.
Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team.
Emergence of a novel swine-origin influenza A (H1N1) virus in humans.
N
Engl J Med 2009;360(25):2605-15.
[Abstract]
[Free
Full Text]
10. Nakajima
S, Nakamura K, Nishikawa F, Nakajima K. Genetic relationship between
the HA genes of type A influenza viruses isolated in off-seasons and
later epidemic seasons. Epidemiol
Infect 1991;106(2):383-95.
[Abstract]
[Free
Full Text]
11. Hope-Simpson RE,
Golubev DB. A new concept of the epidemic process of influenza A
virus. Epidemiol
Infect 1987;99(1):5-54.
[Abstract]
[Free
Full Text]
12. Huang AS,
Baltimore D. Defective viral particles and viral disease processes.
Nature
1970;226(5243):325-7.
[Citation]
13. Nayak DP,
Chambers TM, Akkina RK. Defective-interfering (DI) RNAs of influenza
viruses: origin, structure, expression, and interference. Curr
Top Microbiol Immunol 1985;114:103-51.
[Citation]
14. Henle W, Henle
G. Interference
between inactive and active viruses of influenza. I. The incidental
occurrence and artificial induction of the phenomenon.
Am
J Med Sci 1944;207:705-717.
[Abstract]
15. von Magnus P.
Incomplete forms of influenza virus. Adv Virus Res
1954;2:59-79.
16. Huang AS.
Defective interfering viruses. Annu
Rev Microbiol 1973;27:101-17.
[Citation]
17. Perrault J.
Origin and replication of defective interfering particles. Curr
Top Microbiol Immunol 1981;93:151-207.
[Citation]
18. Roux L, Simon
AE, Holland JJ. Effects
of defective interfering viruses on virus replication and
pathogenesis in
vitro and
in
vivo.
Adv
Virus Res 1991;40:181-211.
[Abstract]
19. Simon AE,
Roossinck MJ, Havelda Z. Plant virus satellite and defective
interfering RNAs: new paradigms for a new century. Annu
Rev Phytopathol 2004;42:415-37.
[Abstract]
20. Chen T, Hudnall
SD. Anatomical mapping of human herpesvirus reservoirs of infection.
Mod
Pathol 2006;19(5):726-37.
[Abstract]
[Free
Full Text]
21. De BK, Nayak DP.
Defective interfering influenza viruses and host cells: establishment
and maintenance of persistent influenza virus infection in MDBK and
HeLa cells. J
Virol 1980;36(3):847-59.
[Abstract]
22. Frielle DW,
Huang DD, Youngner JS. Persistent infection with influenza A virus:
evolution of virus mutants. Virology
1984;138(1):103-17.
[Abstract]
23. Holland JJ,
Villarreal LP. Persistent noncytocidal vesicular stomatitis virus
infections mediated by defective T particles that suppress virion
transcriptase. Proc
Natl Acad Sci U S A 1974;71(8):2956-60.
[Abstract]
[Free
Full Text]
24. Jacobsen FM,
Wehr TA, Sack DA, James SP, Rosenthal NE. Seasonal affective
disorder: a review of the syndrome and its public health
implications. Am
J Public Health 1987;77(1):57-60.
[Abstract]
[Free
Full Text]
25. Wirz-Justice A,
Bucheli C, Graw P, Kielholz P, Fisch HU, Woggon B. Light treatment of
seasonal affective disorder in Switzerland. Acta
Psychiatr Scand 1986;74(2):193-204.
[Abstract]
26. Bellocq C,
Mottet G, Roux L. Wide occurrence of measles virus subgenomic RNAs in
attenuated live-virus vaccines. Biologicals
1990;18(4):337-43.
[Abstract]
27. McLaren LC,
Holland JJ. Defective interfering particles from poliovirus vaccine
and vaccine reference strains. Virology
1974;60(2):579-83.
[Citation]

